Ozone Depletion
Web Module
Anjan K. Chakrabarti
Alejandra De Obeso
Dr. Nihat M. Gürmen
Prof. H. Scott Fogler
The Kinetics of Ozone Depletion
A Numerical Analysis of the Effects of
Catalytic Reactions on Stratospheric Ozone
![]()
![]()
![]()
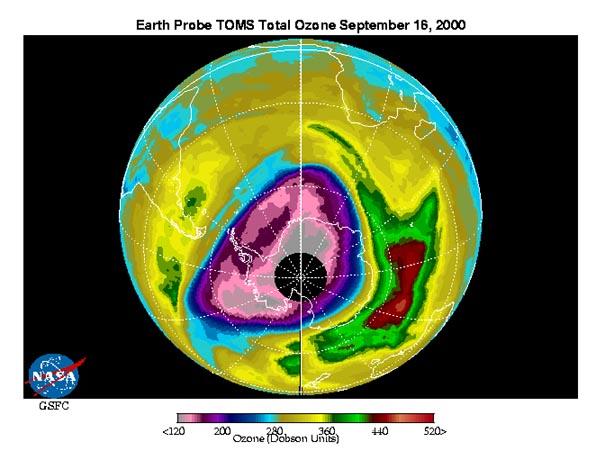
Image Courtesy of NASA
http://jwocky.gsfc.nasa.gov/multi/multi.html
There is a significant hole in the ozone layer over Antarctica, due to several chemical and geophysical conditions. This module will focus on the influence of CFCs, NOx, and Br radicals in the final concentration of O3 molecules in the stratosphere.
Refrigerants, coolants, and propellants for aerosol cans - these all contain and release chlorofluorocarbons (CFCs). Through photodissociation and other mechanisms, chlorine is released into the atmosphere, leading to the catalytic destruction of ozone molecules. Nitrogen oxides (NOx) are also emitted through incomplete combustion of fuels, while bromine free radicals frequently reach the stratosphere through naturally-occurring emissions and human action.
As more and more ozone is depleted in the atmosphere, ultraviolet radiation is able to penetrate the Earth’s surface, leading to such harmful effects as skin cancer and retinal damage. The module will graphically explain how several reaction mechanisms interact with each another, provoking the catalytic destruction of ozone due to free radicals, by applying a pseudo-steady-state hypothesis to this model.