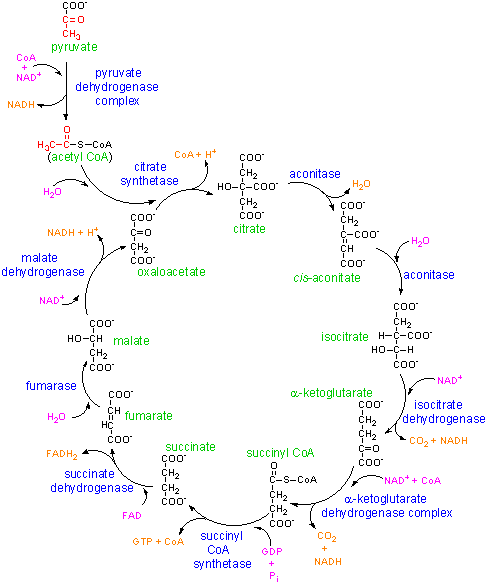

The above picture should be accredited to Georgia State University Chemistry Department; see citations.
The Krebs Cycle or Citric Acid Cycle (CAC)
From glycolysis, pyruvate is produced. This is a small molecule and it is hard for enzymes to grab onto it and also manlipulate it as well. Thus this could be one reason why the Citric Acid Cycle or Krebs Cycle was evolved. A much larger molecule- Coenzyme A (from vitamin pantothenate or B3) is attached to the smaller pyruvate under going manlipulation in Step Zero. Specifically at Step Zero, this molecule is undergoing oxidative decarboxylation or losing a CO2 and being oxidized (losing a pair of electrons). The CO2 is eventually transported out of the aerobically respirating organism. The extra pair of electron will eventually reduce NAD+ (from vitamin niacin) to NADH using detours through TPP (from vitamin thiamine), oxidizd lipoate (attached to the Nitrogen of a Lysine amino acid of a complex of enzymes; also refer to as lipoamide or lipoyllysine), FAD (from vitamin riboflavin or B2).The acetyl CoA then enters the Krebs Cycle. Note 2 points: 1. This step uses FIVE enzyme helpers: FAD, NAD+, lipoate, TPP, and CoA. Four of these come from vitamins so make sure you're not just drinking caffeine free diet coke everyday. 2. This step is analogous to Step Four. The cofactors are the same, the process is the same and the enzymes are extremely similar, indicating a common evolutionary branch.
The Net Equation: Acetyl-S-CoA + 4 oxidized coenzymes[3 NAD+ + 1 FAD] + ADP + Phosphate--> 2 CO2 + 1 ATP + CoA-SH + 4 reduced coenzymes[3 NADH + 1 FADH2]
There are only 4 types of reactions in the Krebs Cycle: Condensation, Hydration/Dehydration, Redox, and Substrate level phosphorylation. All enzymes/coenzymes can be found in the mitochrondia of eukaryotes and the cycle takes place in the matrix of the mitochrondia.
The acetyl group is dropped off into a 4-C molecule using a molecule of H2O (Step One). The new 6-C molecule will rearrange itself by dehydration then hydration (Step 2). The molecule is dehydrogenated twice in two steps (Steps Three and Four). Both dehydrogenation involves the leaving of a CO2 molecule and relocation of an electron pair to NAD+. The latter step is similar to Step Zero- CoA ends up attached to the now 4-C molecule. At the point of attachment is a thioester with a high energy of hydrolysis. Step Five releases that energy and recharges an ADP to ATP with substrate-level phosphorylation. Steps 6-8 involve returning the molecule into Oxaloacetate. Step 6 and 8 are dehydrogenation reactions. Step 7 is a hydration.
Disclaimers/Appendix
Reversible reactions are possible in many of these steps. These are: 2, 5, 6, 7, 8
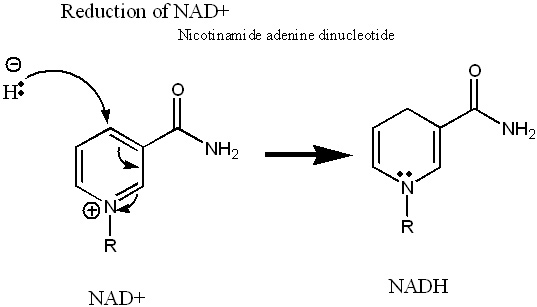
This is the reduction mechanism of NAD/NADH. Oxidation would be just the opposite.
IUPAC Nomenclature
We named as many as possible:
| Biochemistry Name | IUPAC name |
| citrate | 3-Carboxy-3-hydroxypentanedioic acid |
| cis-aconitate | 3-Carboxypent-2-enedioic acid |
| isocitrate | 3-Carboxy-2-hydroxypentanedioic acid |
| alpha-ketoglutarate or oxoglutarate | 2-Oxopentanedioic acid |
| succinyl CoA | CoA-ylsulfanylcarbonylpropanoic acid ??? Total guess |
| succinate | Butanedioic acid |
| fumarate | But-2-enedioic acid |
| malate | 2-Hydroxybutanedioic acid |
| oxaloacetate | 2-Oxobutanedioic acid. Note: IUPAC naming is oxo- but the biochem common name starts with oxa- |
Citations/Links
Nelson, Cox; Lehininer Principles of Biochemistry, 3rd ed. 2000, Worth Publishers, NY.
Georgia State University Chemistry Department
http://chemistry.gsu.edu/glactone/PDB/Proteins/Krebs/Krebs.html
Alberts, Johnson, Lewis, Raff, Roberts, Walter Access Excellence About Biotech.
1998
http://www.accessexcellence.org/AB/GG/citric_Cycle_a.html
Purdue University Chemistry Department
http://chemed.chem.purdue.edu/organic/orgapp/body_energy/citric_acid/step_0_of_citric_acid_cycle.html
Department of Biological Sciences at Carnegie Mellon University
http://info.bio.cmu.edu/Courses/BiochemMols/TCACycle/TCAMain.htm
Rivera, R. Bug Journal 2001, 4, 207-212.